Weather Dependency of Vacuum Systems
Summer is approaching, temperatures are rising. Thunderstorms are in the air, humidity reaches uncomfortable highs. Many people experience problems, especially in sultry weather, i.e., warm and humid air. But what about vacuum systems?
Why would a vacuum system depend on the weather?
In the following article, we'll explain the physical relationships and discuss potential remedies, measures, and limitations.
Problem:
Operators of nearly all vacuum systems are familiar with the following phenomenon. With the arrival of summer, not only do temperatures rise, but also the pumping times of vacuum systems increase significantly. The extent of this increase depends on various factors, such as:
- the size of the volume to be pumped,
- the pumping capacity of the vacuum pumps,
- the temperature of the vacuum system,
- the required operating pressure of the vacuum application, but especially
- the ambient temperature and
- humidity.
This article aims to explain the weather dependency, primarily attributed to humidity and temperature. The other factors mentioned scale the occurring issues to varying degrees:
- The greater the volume of gas pumped per unit of time, the higher the absolute humidity.
- The larger the pumping capacity of a vacuum pump, the greater the moisture tolerance generally, which means the problems occur later or to a lesser extent.
- The lower the required operating pressure of the vacuum application, the more significant the effects of the weather on the pumping times.
Physical Background:
Dry air consists, in simplified terms, of about 78% nitrogen (N2), about 21% oxygen (O2), and about 1% various noble gases, nitrogen oxides, and carbon oxides. Additionally, the air can contain a certain amount of water vapor, depending on the air or surface temperature. Further, the thermal equilibrium case is considered, meaning the air and surface temperatures are identical.
At any water surface, water molecules transition from the water volume into the overlying air volume, i.e., water evaporates. The number of water molecules crossing per unit time is the evaporation rate, mainly determined by the temperature of the water surface.
Simultaneously, water molecules from the air volume collide with the water surface and condense there. This condensation rate, i.e., the number of water molecules returning to the water volume per unit time, depends only on the water vapor partial pressure in the air.
In a system with constant temperature and initially dry air, an evaporation rate corresponding to the surface temperature is established, while the condensation rate is initially zero. Due to continuous evaporation, the number of water molecules in the air, i.e., the water vapor partial pressure, increases, and thus the condensation rate increases. This continues until the system reaches equilibrium, i.e., until the evaporation rate and condensation rate are equal. The concentration of water molecules in the air at equilibrium is referred to as the saturation concentration.
As the temperature increases, the evaporation rate increases, leading to an increase in the water vapor partial pressure in the air, and the condensation rate also increases until a new equilibrium with a higher saturation concentration is established. The magnitude of the saturation concentration is determined solely by the temperature of the water surface, not by the temperature of the air.
The magnitude of the saturation vapor pressure psat,w above a water surface can be calculated using the Magnus equation in the range of -50°C to +100°C.
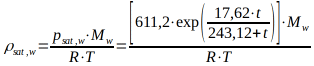
with the saturation vapor pressure psat,w in [Pa] and the temperature ? in [°C].
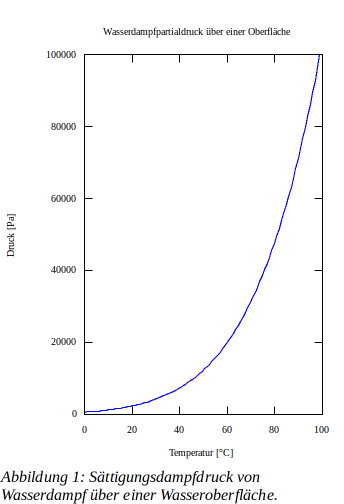
Figure 1 shows the saturation vapor pressure for water vapor above a water surface.
The saturation concentration or saturation density ρsat,w\rho_{\text{sat},w}ρsat,w of water vapor in the air corresponds to the maximum amount of water vapor that an air volume can hold at a certain temperature.
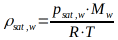
with the saturation density \( \rho_{\text{sat},w} \) in [kg/m3], the molar mass of water \( M_w = 18.02 \times 10^{-3} \) kg/mol, the absolute temperature \( T \) in [K], and the universal gas constant \( R = 8.314 \) J/(mol·K).
The evaporation rate of water is physically limited by the binding forces, hence equilibrium processes after temperature changes take relatively long, which is why the water vapor content of the air almost always exhibits only partial saturation. The percentage of this partial saturation relative to full saturation is referred to as relative humidity.
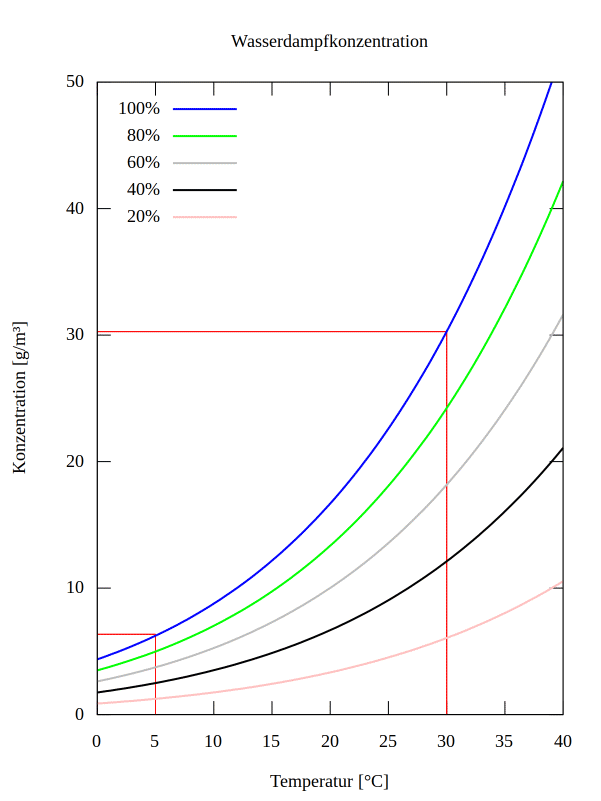
Figure 2 shows the saturation concentration [100%], as well as several partial saturation concentrations for water vapor in the air as a function of the surface temperature.
Effects:
To understand the effects of water vapor concentration in the air on the pumping behavior of vacuum systems, it must be considered that this process can also be viewed in reverse. Warm, saturated air condenses on cooler surfaces, transferring heat until equilibrium is restored. A cooler moisture film forms on the cooler surface. The condensation rate gradually decreases until equilibrium is reached again.
Condensation processes are rapid due to the relatively low heat capacity of air, meaning surfaces quickly accumulate moisture. And all this moisture must be removed during the pumping process.
To illustrate the differences, here are two example calculations for the amount of water in the air, once for a day in winter and once in midsummer.
For the water vapor saturation concentration:
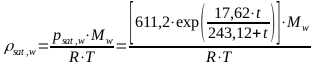
In winter, the temperature is \( t = 5°C \), so the absolute temperature is \( T = 278K \). This yields a saturation density value of \( \rho_{\text{sat},w} = 6.80 \, \text{g/m}^3 \) (see Figure 2).
In summer, the temperature is \( t = 30°C \), and the absolute temperature is \( T = 303K \). This results in a saturation density value of \( \rho_{\text{sat},w} = 30.28 \, \text{g/m}^3 \) (see Figure 2).
Assuming that the surface of the vacuum chamber remains at a constant temperature, we can see that in summer, the amount of water vapor introduced is 4.5 times greater.
If condensation occurs in the vacuum system, the pumping time immediately increases significantly. Vacuum pumps can only convey gaseous substances, meaning all condensed water must be returned to the vapor phase. However, the evaporation cools the surface further, causing the evaporation rate to decrease even more.
The pressure in the vacuum chamber decreases as long as water or ice is present, analogous to the temperature according to Figure 1.
Another type of vacuum system is short-cycle systems that vent to atmosphere and then evacuate again at relatively short intervals. The pumped gas volume is quite high, meaning a significantly higher water vapor content is pumped in summer. This can lead to condensation of water both in the vacuum system and in the vacuum pump, resulting in a significant increase in base pressure and further extensions of the pumping time.
Remedies:
Possible measures against weather-related increases in pumping time include:
- Increasing the surface temperature in the vacuum chamber during ventilation.
- Ventilation with already dried air.
- Use of a cold trap with a refrigeration unit to increase water vapor pumping capacity (more on this in a forthcoming article).
To protect the vacuum pumps from accumulation of condensed water, gas ballast can be used. Possible applications, limitations, and potential limitations imposed by the vacuum pumps used will be discussed in the upcoming article.

